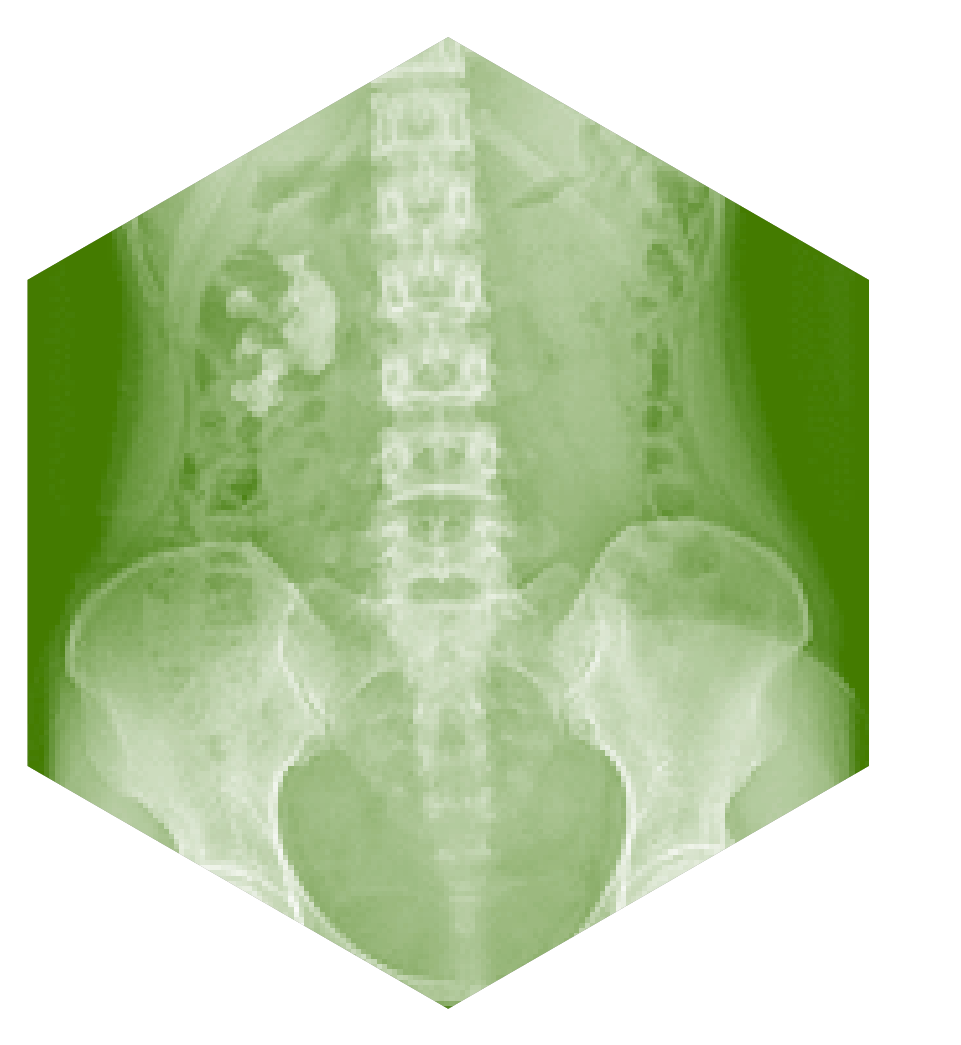
Cystinuria is caused by faulty genes that cause the kidneys to leak amino acids into the urine. This problem in amino acid retention causes high concentrations of an amino acid called cystine in the urine. This crystallises, as large crystal-stones, in the kidneys. These crystal-stones block the flow of urine, causing pain and infection. Surgery is often the only way of removing them. Damage to the kidneys caused by surgery, and repeated infection can cause progressive and permanent kidney failure. Severely affected patients can have many kidney stone events each year, causing significant disruption to work, family life as well as the education of younger patients.
There are about 45,000 in the US and 100,000 people in the EU with Cystinuria; most people have their first experience of cystine crystal-stones causing pain or other symptoms in their teens
or early 20’s.
There have been no new treatments introduced for over 30 years for people with Cystinuria.
Current treatments to prevent cystine stones are effective in around half of the patients that take them. These older treatments have significant side effects, including skin rashes, liver and
bone marrow disorders.
The experiences of people with cystinuria and discussion on current and new potential treatments have been featured in the Spring 2018 edition of the digital magazine RareRevolution here.